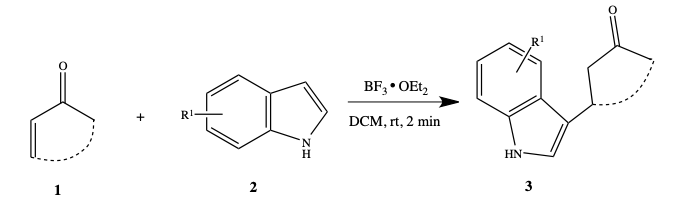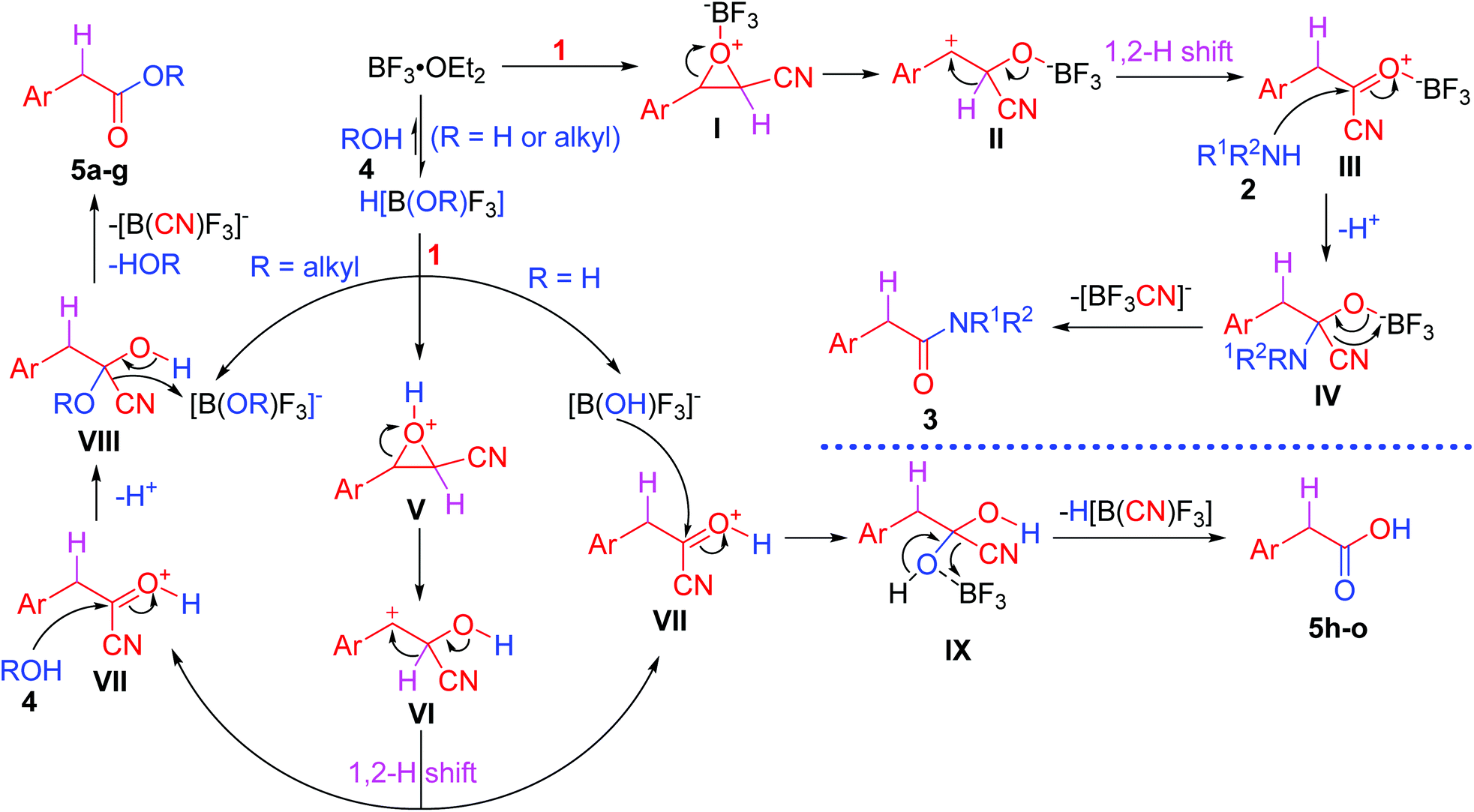
BF 3 ·OEt 2 -promoted tandem Meinwald rearrangement and nucleophilic substitution of oxiranecarbonitriles - Organic & Biomolecular Chemistry (RSC Publishing) DOI:10.1039/C9OB02428J

Revisit of the phenol O-glycosylation with glycosyl imidates, BF3·OEt2 is a better catalyst than TMSOTf - ScienceDirect

Lewis acid BF3·OEt2-catalyzed Friedel–Crafts reaction of methylenecyclopropanes with arenes - ScienceDirect

Glycosyl Fluorides as Intermediates in BF3·OEt2‐Promoted Glycosylation with Trichloroacetimidates - Nielsen - 2017 - European Journal of Organic Chemistry - Wiley Online Library
![Scheme 4. Plausible Mechanism for Formation of Pyrazino[1,2-a]indoles... | Download Scientific Diagram Scheme 4. Plausible Mechanism for Formation of Pyrazino[1,2-a]indoles... | Download Scientific Diagram](https://www.researchgate.net/profile/Imtiyaz-Wani/publication/328821710/figure/fig1/AS:715234343931905@1547536564808/Scheme-4-Plausible-Mechanism-for-Formation-of-Pyrazino1-2-aindoles-and.png)
Scheme 4. Plausible Mechanism for Formation of Pyrazino[1,2-a]indoles... | Download Scientific Diagram

PDF) BF3·OEt2‐Promoted Tandem O‐Arylation/Hydroxylation: Efficient Synthesis of 2‐Hydroxyflavanones from Triazenylaryl‐Substituted Diketones
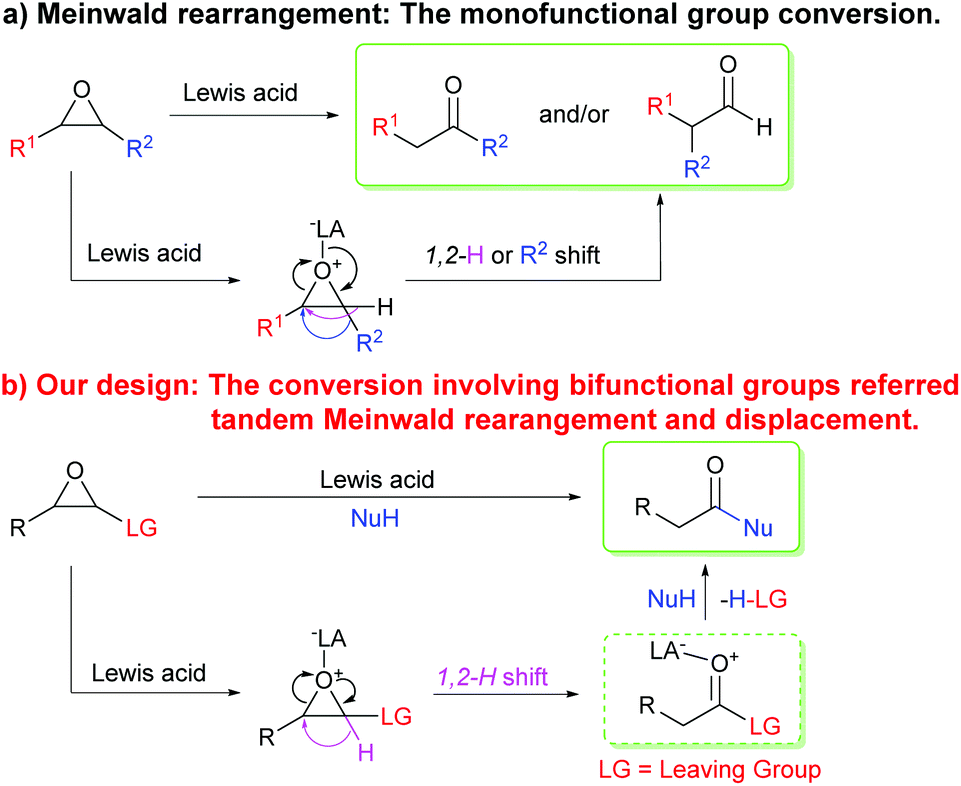
BF 3 ·OEt 2 -promoted tandem Meinwald rearrangement and nucleophilic substitution of oxiranecarbonitriles - Organic & Biomolecular Chemistry (RSC Publishing) DOI:10.1039/C9OB02428J

Glycosyl Fluorides as Intermediates in BF3·OEt2‐Promoted Glycosylation with Trichloroacetimidates - Nielsen - 2017 - European Journal of Organic Chemistry - Wiley Online Library

PIFA–BF3·OEt2 mediated intramolecular regioselective domino cyclization of ynamides: A novel method for the synthesis of tetrahydroisoquinoline-oxazol-2(3H)-ones - ScienceDirect

BF3·OEt2-promoted concise synthesis of difluoroboron-derivatized curcumins from aldehydes and 2,4-pentanedione - ScienceDirect

BF3·OEt2-Mediated Highly Regioselective SN2-Type Ring-Opening of N-Activated Aziridines and N-Activated Azetidines by Tetraalkylammonium Halides | The Journal of Organic Chemistry
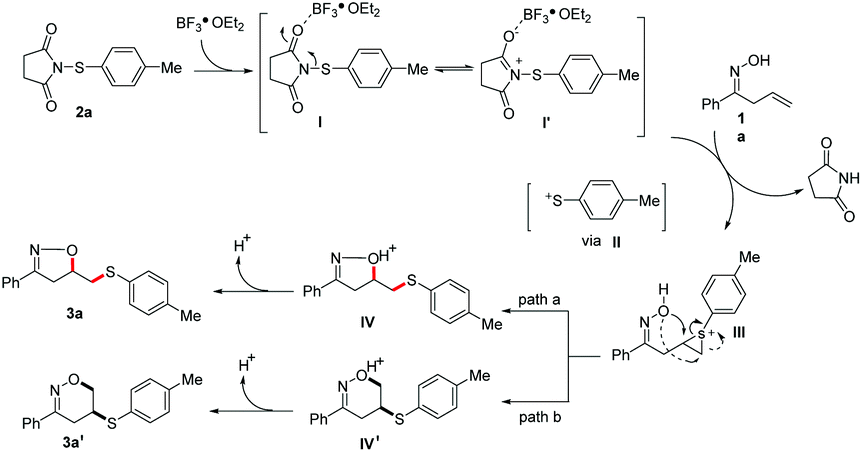
BF 3 ·OEt 2 -mediated cyclization of β,γ-unsaturated oximes and hydrazones with N -(arylthio/arylseleno)succinimides: an efficient approach to synthes ... - Organic & Biomolecular Chemistry (RSC Publishing) DOI:10.1039/D0OB01388A