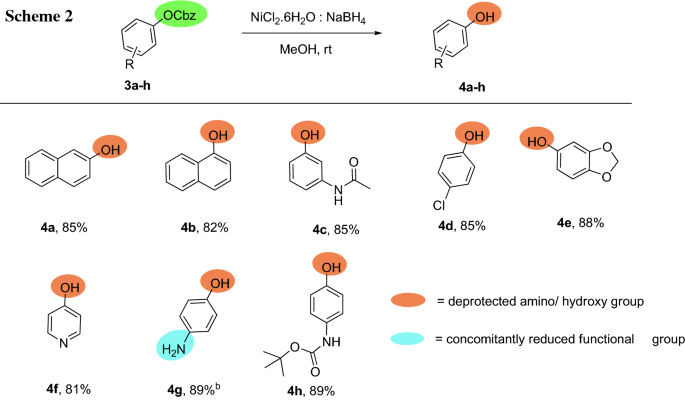
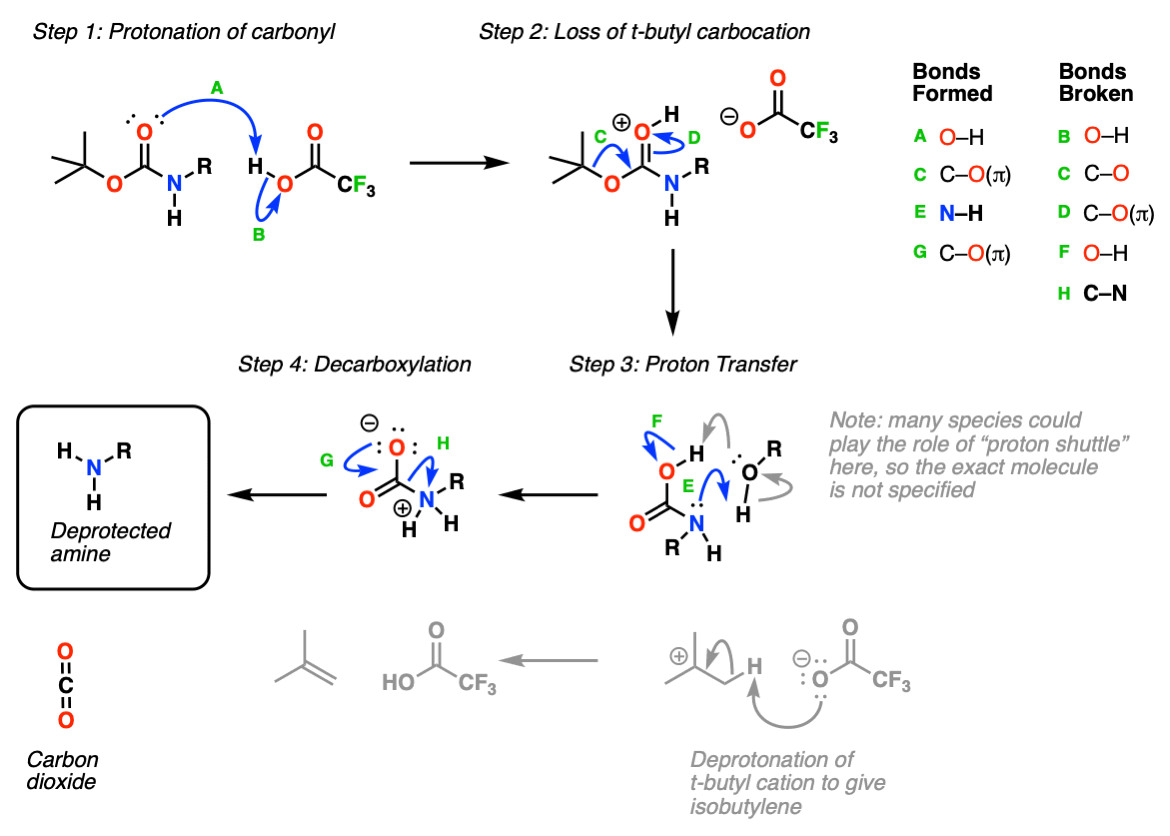
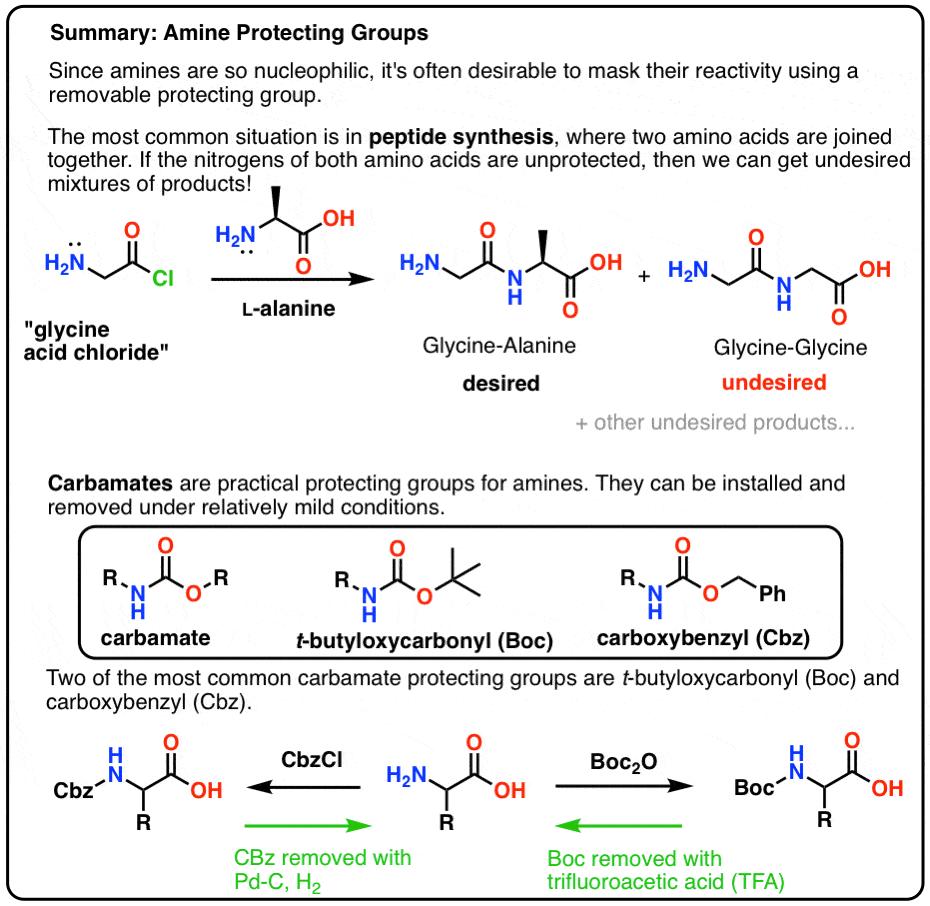
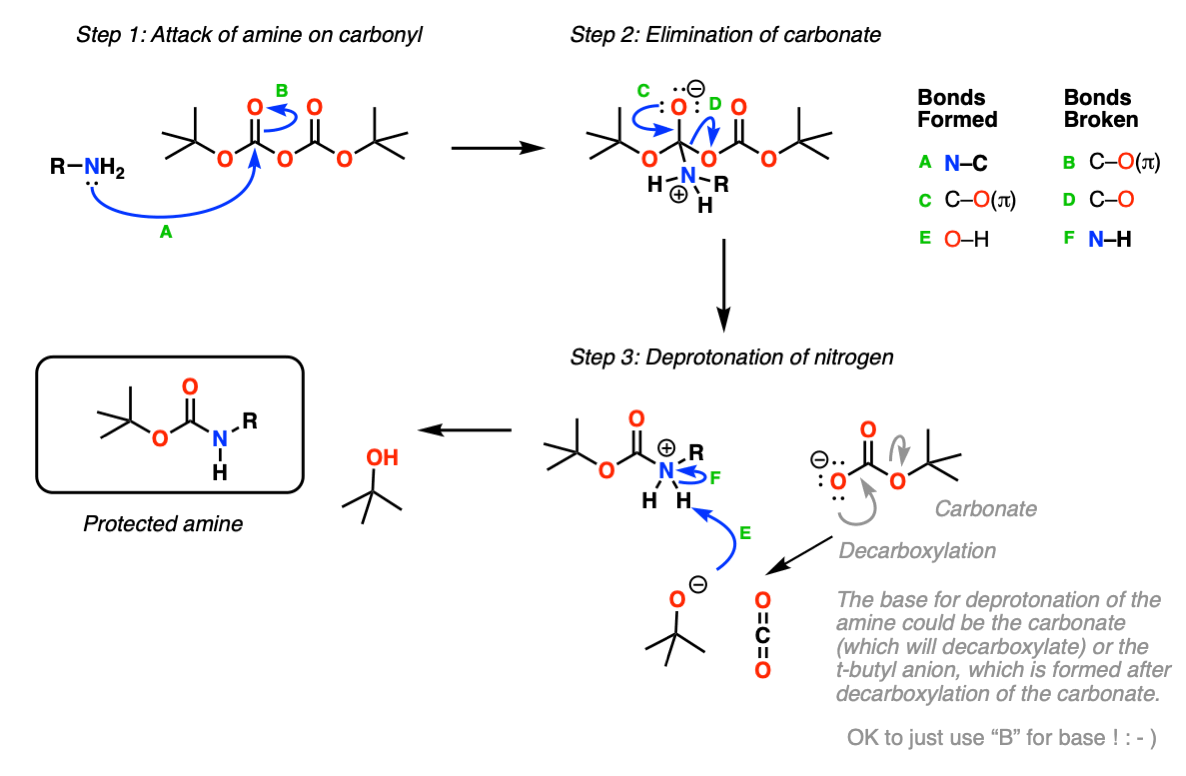
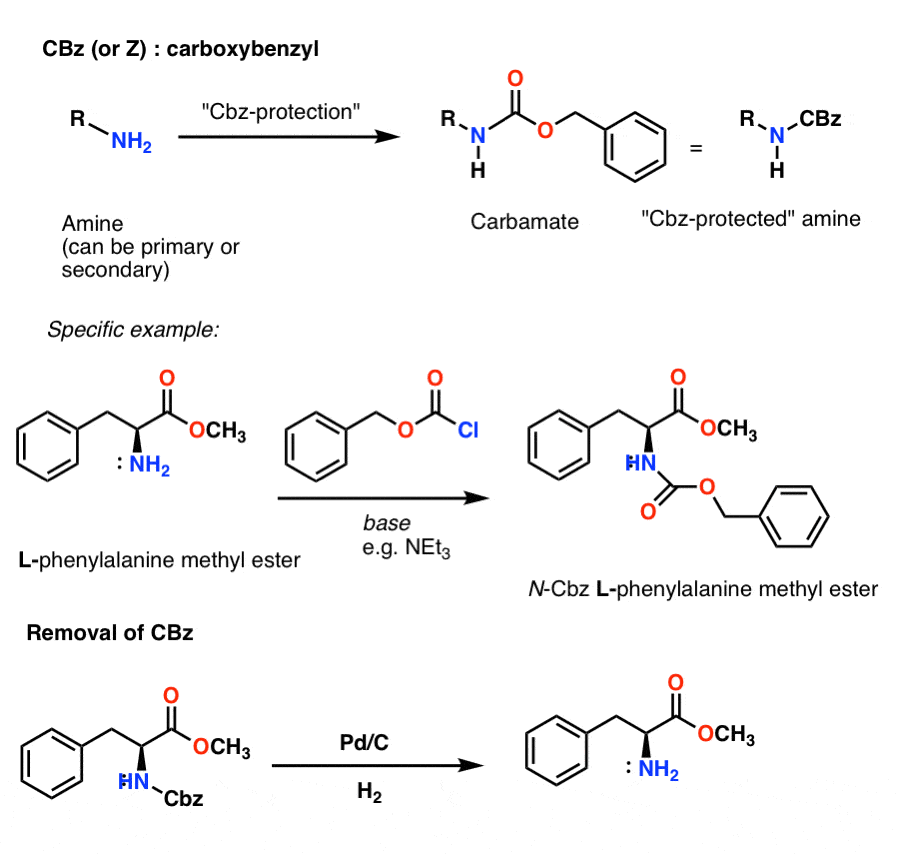
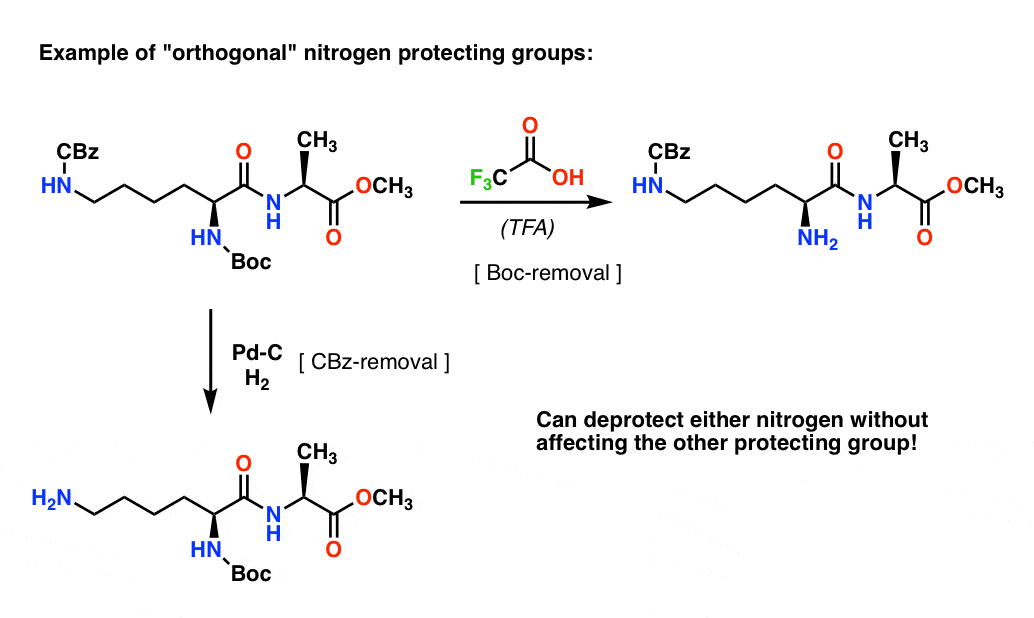
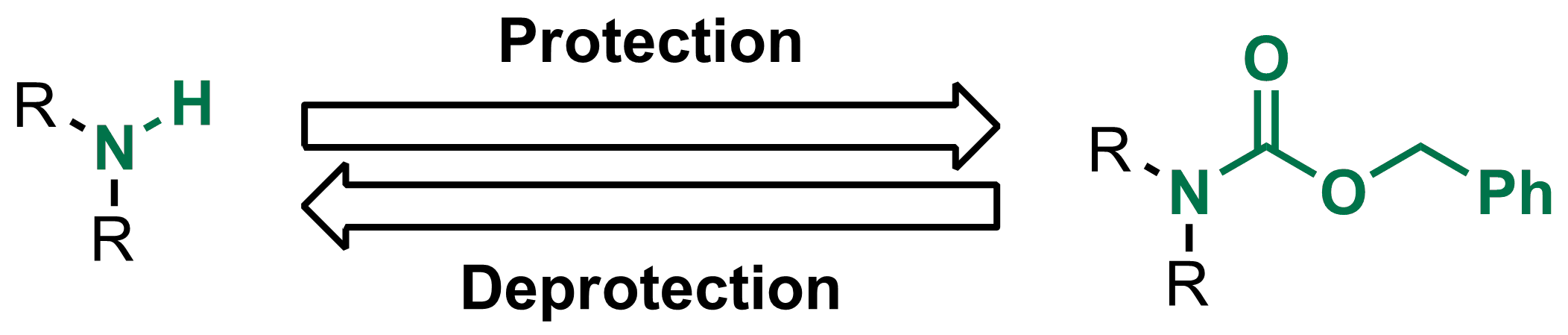
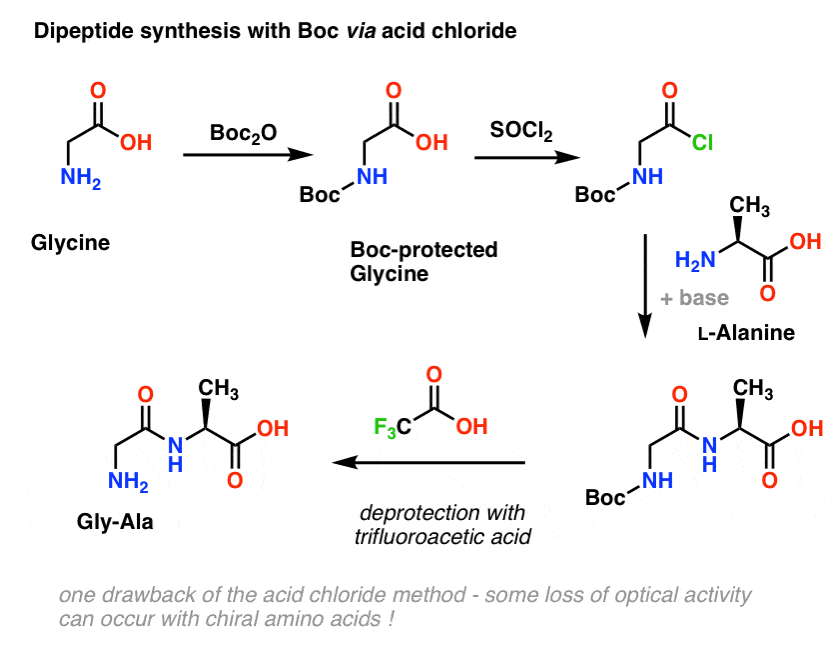
organic chemistry - Deprotection of carboxybenzyl (Cbz) in the presence of Methyl ester? - Chemistry Stack Exchange
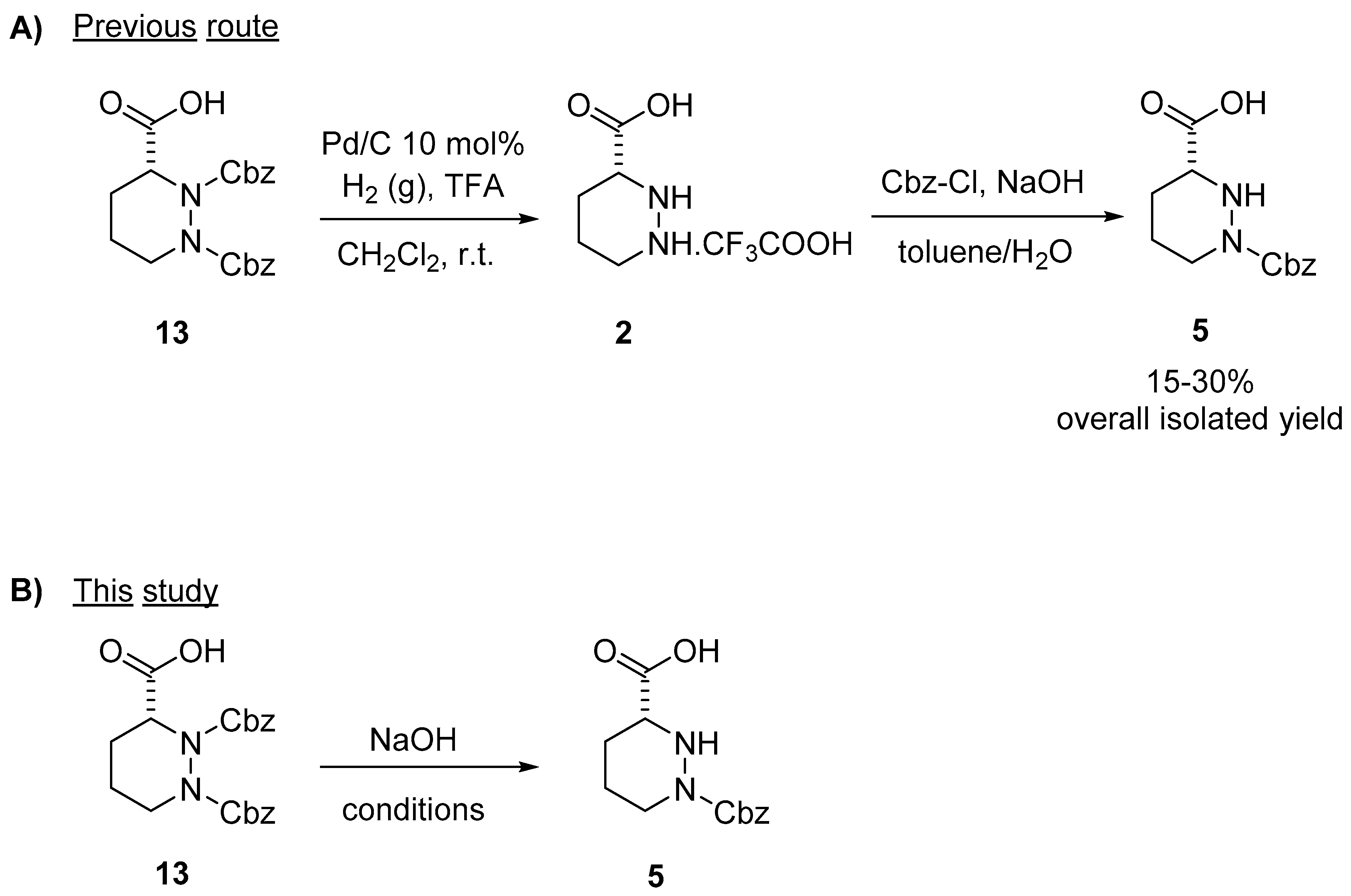
Molecules | Free Full-Text | A Reliable Enantioselective Route to Mono-Protected N1-Cbz Piperazic Acid Building Block | HTML

Development of a novel protocol for chemoselective deprotection of N/O-benzyloxycarbonyl (Cbz) at ambient temperature | SpringerLink

Molecules | Free Full-Text | A Reliable Enantioselective Route to Mono-Protected N1-Cbz Piperazic Acid Building Block | HTML

Tertiary-butoxycarbonyl (Boc) – A strategic group for N-protection/ deprotection in the synthesis of various natural/unnatural N-unprotected aminoacid cyanomethyl esters - ScienceDirect

A convenient protocol for the deprotection of N-benzyloxycarbonyl (Cbz) and benzyl ester groups - ScienceDirect

Selective deprotection of the Cbz amine protecting group for the facile synthesis of kanamycin A dimers linked at N-3″ position - ScienceDirect

A convenient protocol for the deprotection of N-benzyloxycarbonyl (Cbz) and benzyl ester groups - ScienceDirect
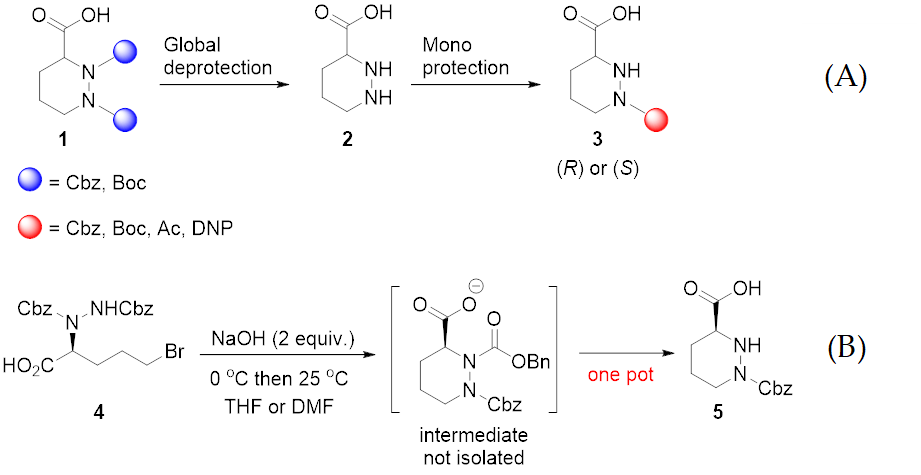
Molecules | Free Full-Text | A Reliable Enantioselective Route to Mono-Protected N1-Cbz Piperazic Acid Building Block | HTML