Solved] 16b The effective rate constant for a gaseous reaction that has a Lindemann-Hinshelwood mechanism is 1.7 x 10 3s at 1.09 kPa and 2.2 x 10 #s... | Course Hero
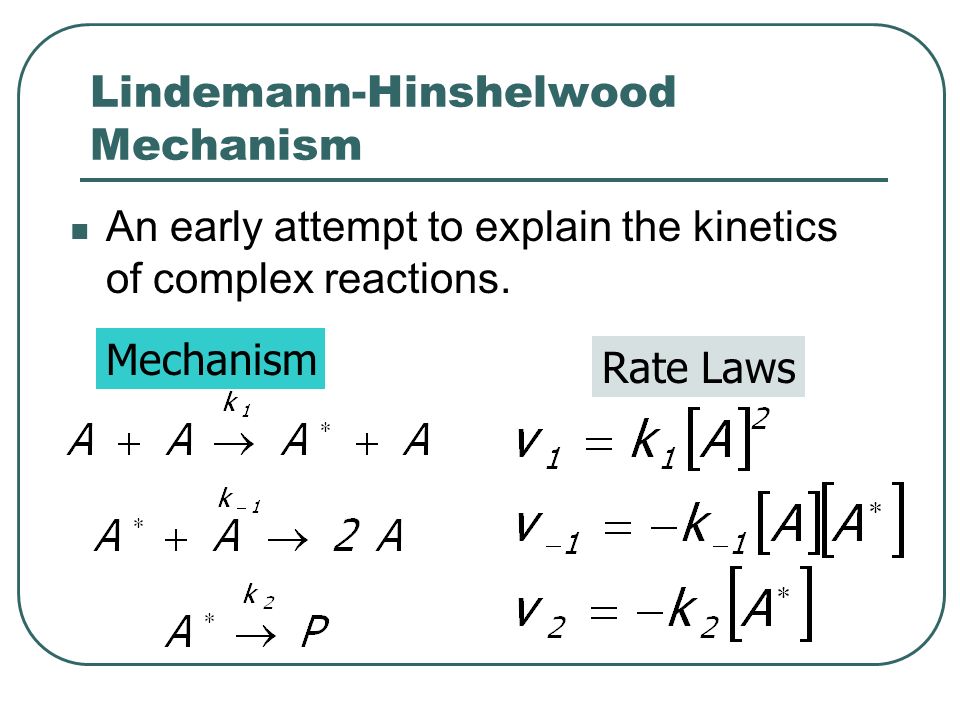
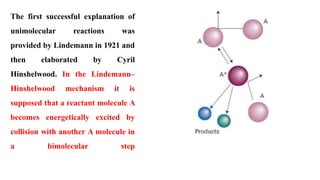
Chemistry 232 Complex Reaction Mechanisms. Lindemann-Hinshelwood Mechanism An early attempt to explain the kinetics of complex reactions. Mechanism Rate. - ppt download
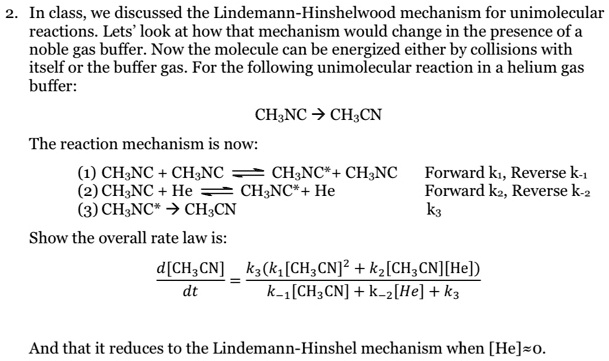
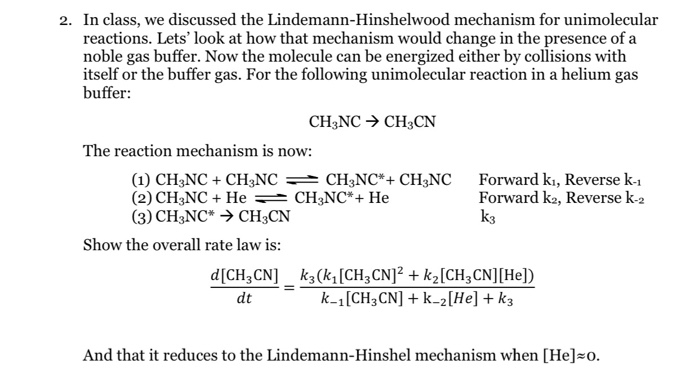
SOLVED:In class_ we discussed the Lindemann-Hinshelwood mechanism for unimolecular reactions. Lets' look at how that mechanism would change in the presence of a noble gas buffer: Now the molecule can be energized

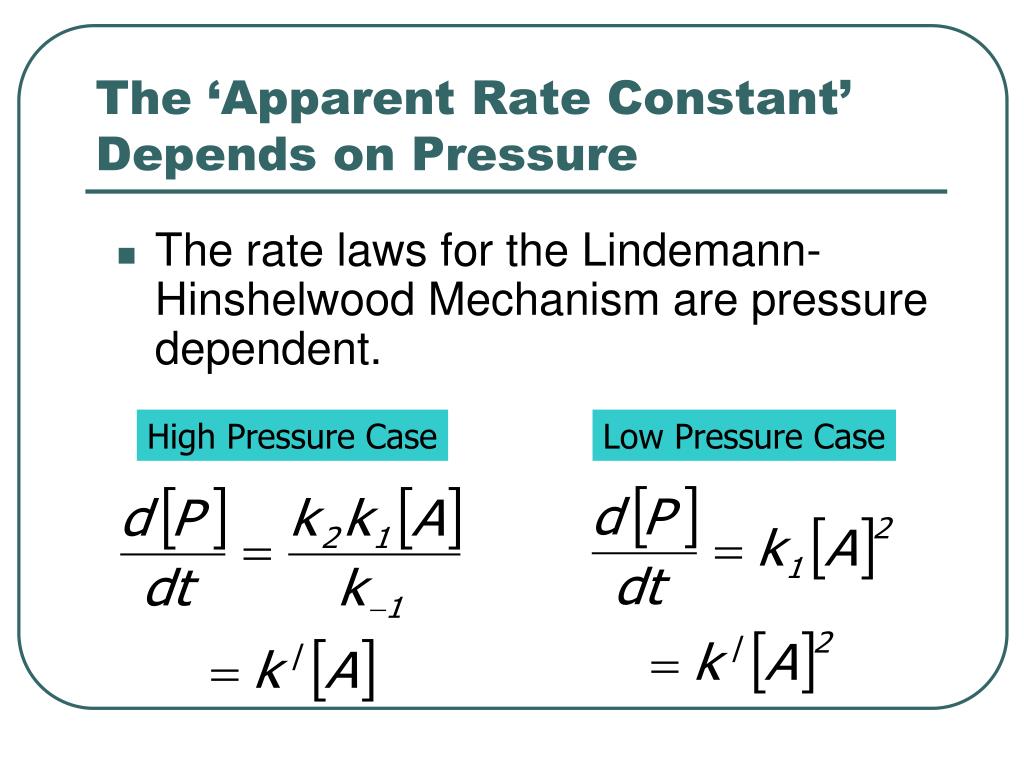
How/Why have the arranged the Lindemann-Hinshelwood mechanism like this for 2 pressures (3rd line? : r/chemhelp
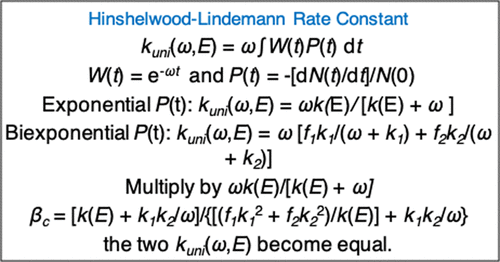
Comparison of Exponential and Biexponential Models of the Unimolecular Decomposition Probability for the Hinshelwood–Lindemann Mechanism,The Journal of Physical Chemistry Letters - X-MOL
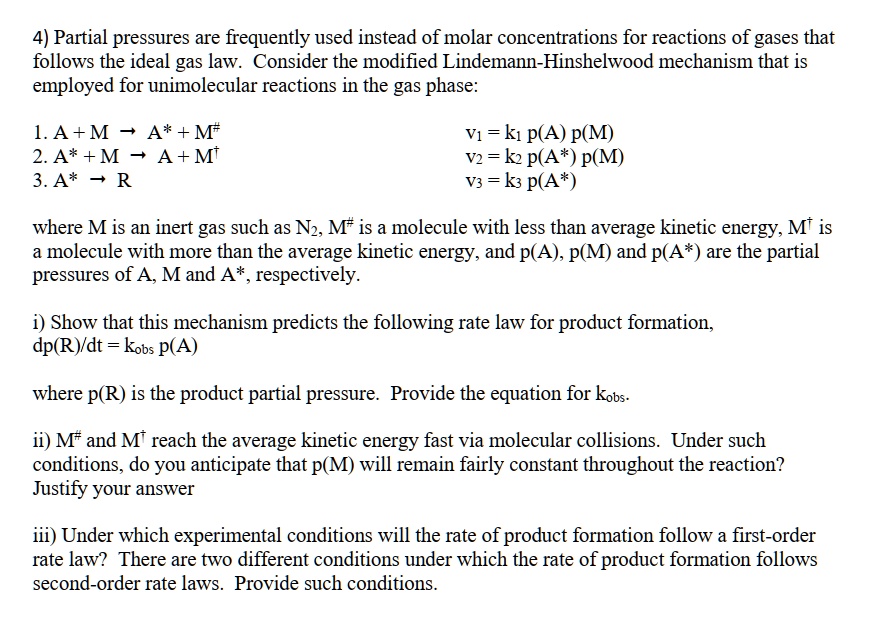
SOLVED:4) Partial pressures are frequently used instead of molar concentrations for reactions of gases that follows the ideal gas law. Consider the modified Lindemann-Hinshelwood mechanism that is employed for unimolecular reactions in
![SOLVED:Question 5 1pts Lindemann-Hinshelwood Mechanism (1) A+A->A+A '2) A*+A~>A+A '3) A" Which step activation? [Choosc | Which stcp deactivation? Choosc Which stcp decay? Choosc ] Atvery high pressure; d[PVdt follows what order SOLVED:Question 5 1pts Lindemann-Hinshelwood Mechanism (1) A+A->A+A '2) A*+A~>A+A '3) A" Which step activation? [Choosc | Which stcp deactivation? Choosc Which stcp decay? Choosc ] Atvery high pressure; d[PVdt follows what order](https://cdn.numerade.com/ask_images/2aa8572d4e754303a2d8cd4d4b687a8d.jpg)
SOLVED:Question 5 1pts Lindemann-Hinshelwood Mechanism (1) A+A->A+A '2) A*+A~>A+A '3) A" Which step activation? [Choosc | Which stcp deactivation? Choosc Which stcp decay? Choosc ] Atvery high pressure; d[PVdt follows what order